PlatoScience CEO on why he sees mental health as the future of tDCS devices
Guest Post by Balder Onarheim | PlatoScience Neurostimulation
The Growth of Medical Use of tDCS
While tDCS has historically always been used for medical purposes, for a long time the more ‘performance enhancement’ aspects of tDCS has received more attention – largely due to the 2014 RadioLab episode 9-Volt Nirvana. However, in the more recent years the focus seems to have shifted back to medical use, especially for mental health, but also for a number of other medical purposes such as pain, addiction, and rehabilitation.
The two large independent consensus papers on clinical tDCS, from 2017 and 2021, illustrate the rapid growth in applications with strong evidence:
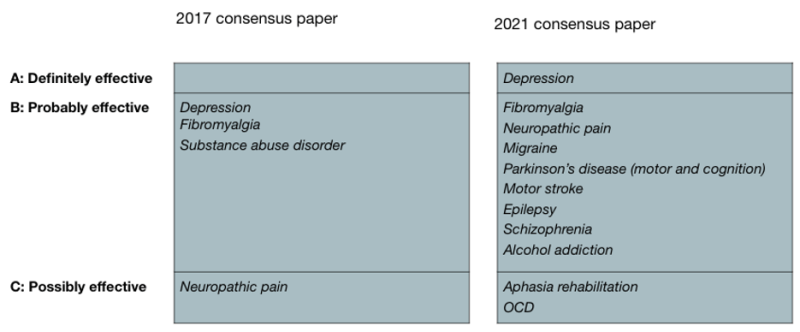
And considering the significant growth in tDCS studies it is safe to assume that a 2023 consensus paper would be likely to see a continued migration from level C, to B, to A.

Since tDCS has an excellent safety profile – a 2016 review found no serious adverse events across more than 30000 stimulation sessions within the standard parameters (see LOTES-2017) – and thus it is very well suited for research on new possible applications. This is especially relevant for conditions where there are few or no effective treatments, or the side effects are so undesirable that patients choose the condition.
For clinicians and their patients, the minimal risks/side effects from tDCS often makes it worthwhile to try tDCS, even as an experimental treatment. For many clinicians we work with, who are often frustrated with the lack of effective treatments at their disposal, tDCS provides a long-awaited new option for large groups of patients.
Remote tDCS
It is no secret that the single-session effect of tDCS is unpredictable, and that the effects are not the same for all users, but both these drawbacks can be evened out by repeated use over time and by monitoring the effects of stimulation and changing the parameters if the desired results cannot be achieved with standard protocols.
The technical simplicity of tDCS makes the equipment comparably inexpensive, and the portability allows for patients to bring devices home for more frequent use – and digital technologies enable live monitoring by clinicians. This way, patients can stimulate as frequently as they like, and their responsible clinician can remotely monitor the compliance and effectiveness, even changing tDCS parameters remotely if needed.
Historically, the studies of tDCS have been largely lab based, which is logistically challenging and expensive, thus studies rarely have more than a handful of sessions, thus the amount of studies of accumulated effects over time has been limited. It has also led to interesting data features such as stimulation often happening five days a week (lab opening hours) instead of seven.
But generally, across long term studies, reviews find that the effects are increasing over time, showing that longer paradigms are more effective, and importantly there is no correlation between longer paradigms and increased adverse event rates.

Finally, due to the lack of approved tDCS equipment specifically designed for remotely monitored use, there have so far been very few structured long term at-home studies where participants are using devices themselves. This means that the expected continuous clinical benefits from patients self administering tDCS at-home with remote clinical supervision, is not yet well documented, while the safety of continued long term use is.
The PlatoWork Headset in Medical Use
Based on the above, the PlatoWork headset has proven to be a very useful device for supporting both clinicians and researchers interested in studying the health benefits of continuous use of tDCS in real world settings. Being one of the two first cloud based tDCS headsets on the market, we have over seven years of experience with self administered at-home use, which has allowed us to fine tune the usability and digital eco system for such use. Also participation in both lab research and collaborations such as the Nissan “Brain to performance project”, has enabled wide user input.
These experiences have allowed PlatoScience to become a key player in the emerging field of at-home medical tDCS. Also the PlatoWork device in itself is an important part of this: the electrode positions are based on the standard 10-20 system, in a fixed headset frame design securing correct electrode placement, and rotation of the headset frame allows for reaching a number of key electrode positions. With its three electrodes, all remotely programmable to work as anode/cathode, different protocols can be configured and the smartphone app also allows researchers to program sham (placebo) sessions.
Opposite to other devices in the field that focus narrowingly on one or two predetermined indications/protocols, PlatoScience’s flexible device supports an approach where clinicians and researchers can include medical tDCS in their treatment and/or studies. We continue to see an increased interest amongst clinicians to better measure the effectiveness of various treatment forms, allowing an emergence of clinician-researchers who’s data collection represents a crucial addition to the old lab study paradigms of tDCS research.
The Future of Non-medical tDCS
As with the studies on medical use of tDCS, also the performance enhancement space has seen a continuous growth in recent years. In my point of view, the findings are less clear and results vary from improvement to clear negative effects (recent example from Nature), and in our experience the inconvenience/benefit ratio was not always acceptable for healthy users even if they experience positive effects at times.
Furthermore, the regulatory space of tDCS is changing. It has already started in the EU, where regulators have ruled that tDCS – regardless of intended use – must be regulated as medical devices. And a December 2021 ruling has gone as far as reclassifying non-medical tDCS devices as Class III, which is the same regulatory class as pacemakers and deep brain stimulation. Many countries have already either committed to adopting the new EU rules (the MDR) directly, or have indicated intentions of following them very closely.
Thus, I expect that providing non-medical tDCS devices directly to consumers will get increasingly complicated in the coming years. As a result, and all the above arguments for the value of tDCS for medical purposes, at the end of 2021 PlatoScience discontinued its B2C offering. We are, of course, continuing to support our previous B2C customers, but will not in the foreseeable future offer devices for non-medical use. As a neurotech founder and headset designer I am very thankful for the years of support and dedication from our users and the tDCS community generally, but personally I believe that the future for tDCS is remotely supervised, at-home, medical use.





